Weed Schedule 3: A Comprehensive Legal Overview
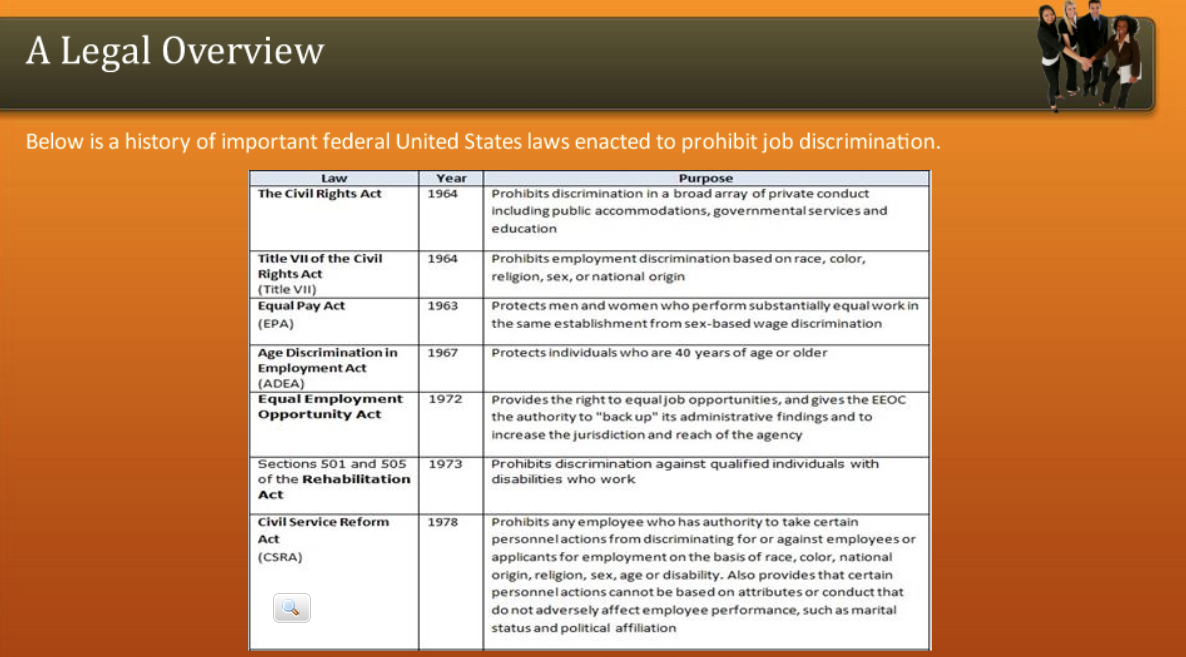
Welcome to a comprehensive guide on Weed Schedule 3, a critical aspect of legal frameworks surrounding cannabis. This article aims to provide an in-depth analysis, exploring the intricacies of Schedule 3 classifications, their legal implications, and the impact on the cannabis industry and its stakeholders.
Understanding Weed Schedule 3: Definitions and Classifications

The term “Weed Schedule 3” refers to a specific category within the broader classification system used to regulate controlled substances, particularly in the context of cannabis legislation. Schedule 3 classifications typically denote substances with recognized medical value but also carry a moderate to low potential for abuse or dependence.
In the United States, the Controlled Substances Act (CSA) establishes five schedules (1 to 5) to categorize drugs and substances based on their accepted medical use, potential for abuse, and safety profiles. Weed Schedule 3, therefore, occupies a unique position within this framework, reflecting a delicate balance between recognizing the therapeutic potential of cannabis while also managing associated risks.
Schedule 3 Criteria and Examples
To be classified as Schedule 3, a substance must meet specific criteria outlined by regulatory bodies. These criteria often include a thorough evaluation of the substance’s pharmacological properties, abuse potential, and therapeutic benefits. Substances in this category typically exhibit a lower risk profile compared to Schedule 2 substances, which are considered more dangerous and prone to abuse.
Examples of substances that fall under Weed Schedule 3 include certain synthetic cannabinoids, such as dronabinol and nabilone, which are prescribed for medical conditions like nausea and vomiting associated with chemotherapy. These substances are often manufactured in controlled environments and are subject to strict regulations regarding production, distribution, and prescription.
| Substance | Medical Use |
|---|---|
| Dronabinol | Treatment of nausea and vomiting caused by chemotherapy |
| Nabilone | Management of severe nausea and vomiting in cancer patients |

Legal Implications and Regulatory Landscape

The legal implications of Weed Schedule 3 classifications are far-reaching, impacting various aspects of the cannabis industry, from cultivation and manufacturing to distribution and patient access.
Cultivation and Manufacturing
For cultivators and manufacturers, Schedule 3 classifications present both opportunities and challenges. On the one hand, the recognition of medical value can open doors to expanded research and development, leading to the creation of innovative cannabis-based products. On the other hand, the strict regulations surrounding Schedule 3 substances can impose significant compliance burdens, including rigorous quality control measures and adherence to Good Manufacturing Practices (GMP) standards.
Distribution and Patient Access
Schedule 3 classifications also influence the distribution and accessibility of cannabis-based medications. In many jurisdictions, these substances are available only through specialized pharmacies or medical dispensaries, ensuring proper patient monitoring and adherence to prescribed dosages. The distribution process often involves rigorous tracking and reporting systems to prevent diversion and ensure responsible use.
Regulatory Challenges and Future Outlook
The regulatory landscape surrounding Weed Schedule 3 is not without its complexities. As the cannabis industry continues to evolve, policymakers and regulatory bodies face the challenge of balancing the need for patient access and medical innovation with the imperative to prevent abuse and ensure public safety. This delicate equilibrium often leads to ongoing debates and revisions of existing regulations.
Looking ahead, the future of Weed Schedule 3 classifications may be shaped by advancements in scientific understanding, changing societal attitudes towards cannabis, and the accumulation of real-world evidence regarding the safety and efficacy of cannabis-based therapies. These factors will likely influence future regulatory decisions and potentially lead to further refinements or adjustments to the Schedule 3 criteria.
Impact on the Cannabis Industry and Stakeholders
The implications of Weed Schedule 3 classifications extend beyond the legal and regulatory realms, significantly impacting various stakeholders within the cannabis industry.
Patient Experience and Access
For patients relying on cannabis-based medications, Schedule 3 classifications can present both advantages and challenges. The recognition of medical value can provide patients with access to a broader range of therapeutic options, potentially improving their quality of life. However, the stringent regulations and distribution channels associated with Schedule 3 substances may create barriers to access, particularly for patients in underserved areas or those facing financial constraints.
Industry Innovation and Development
From a business perspective, Schedule 3 classifications can stimulate innovation and development within the cannabis industry. Recognizing the medical potential of certain cannabis compounds can encourage research and investment in developing new products, formulations, and delivery methods. This, in turn, can drive economic growth, create jobs, and foster a more robust and diverse cannabis market.
Challenges and Opportunities for Stakeholders
However, navigating the complexities of Weed Schedule 3 regulations also presents challenges for industry stakeholders. Compliance with stringent regulations can impose significant costs and operational burdens, particularly for smaller businesses or those lacking the necessary infrastructure. Additionally, the dynamic nature of cannabis regulations can create uncertainty, impacting investment decisions and long-term planning.
Despite these challenges, the opportunities presented by Weed Schedule 3 classifications are significant. By embracing the medical potential of cannabis, industry stakeholders can position themselves at the forefront of a rapidly evolving industry, contributing to advancements in healthcare and patient well-being.
Performance Analysis and Real-World Examples
To provide a more nuanced understanding of Weed Schedule 3 classifications, it is essential to examine real-world examples and performance data. Case studies and empirical evidence can shed light on the practical implications and outcomes associated with Schedule 3 substances.
Case Study: Dronabinol in Cancer Care
Dronabinol, a synthetic cannabinoid classified as Schedule 3, has been extensively studied for its efficacy in managing chemotherapy-induced nausea and vomiting. Numerous clinical trials and real-world evidence have demonstrated its effectiveness in alleviating these symptoms, leading to improved patient outcomes and quality of life. This case study exemplifies how Schedule 3 substances can play a crucial role in evidence-based medicine, offering safe and effective treatment options for patients in need.
Performance Metrics and Patient Outcomes
Analyzing performance metrics and patient outcomes associated with Schedule 3 substances can provide valuable insights into their therapeutic value and impact. Key performance indicators (KPIs) such as patient satisfaction, treatment adherence, and clinical outcomes can be tracked and evaluated to assess the overall effectiveness and safety of these substances. This data-driven approach can inform regulatory decisions, guide industry practices, and ultimately improve patient care.
Evidence-Based Future Implications

As the body of evidence surrounding Weed Schedule 3 substances continues to grow, it is essential to consider the future implications and potential directions for this classification system.
Advancements in Cannabis Research
Ongoing research into the therapeutic potential of cannabis and its constituents is likely to yield new insights and discoveries. As scientific understanding evolves, it may become necessary to reevaluate and refine the criteria for Weed Schedule 3 classifications. This could involve adjusting the risk profiles associated with certain substances or exploring new therapeutic applications beyond those currently recognized.
Shifting Societal Attitudes and Policy Changes
The dynamic nature of societal attitudes towards cannabis and the evolving legal landscape can also influence the future of Weed Schedule 3. As more jurisdictions legalize cannabis for medical and recreational purposes, the regulatory framework may need to adapt to accommodate these changes. This could involve revisiting the Schedule 3 criteria, exploring alternative classification systems, or implementing more flexible regulations to promote innovation and patient access.
International Perspectives and Harmonization
Furthermore, the international perspective on cannabis regulation adds another layer of complexity. As more countries reconsider their approaches to cannabis, there may be opportunities for harmonization and collaboration. International discussions and collaborations can lead to the establishment of global standards and guidelines, ensuring a consistent and evidence-based approach to Weed Schedule 3 classifications across borders.
Conclusion
In conclusion, Weed Schedule 3 classifications play a pivotal role in the legal and regulatory framework surrounding cannabis. This comprehensive guide has explored the definitions, legal implications, and impact on various stakeholders, highlighting the delicate balance between recognizing medical value and managing associated risks. As the cannabis industry continues to evolve, ongoing research, evidence-based decision-making, and a commitment to patient well-being will be crucial in shaping the future of Weed Schedule 3 classifications and the broader cannabis landscape.
What are the key differences between Weed Schedule 2 and Schedule 3 classifications?
+Weed Schedule 2 and Schedule 3 classifications differ primarily in terms of their risk profiles and potential for abuse. Schedule 2 substances are considered more dangerous and prone to abuse, while Schedule 3 substances exhibit a lower risk profile. Schedule 2 substances often require stricter regulations and control measures, while Schedule 3 substances are subject to less stringent regulations but still require careful monitoring and distribution.
How do Schedule 3 classifications impact the development of cannabis-based medications?
+Schedule 3 classifications can stimulate the development of cannabis-based medications by recognizing their medical value. This recognition can attract research and investment, leading to the creation of new products and formulations. However, the stringent regulations associated with Schedule 3 substances can also impose challenges, particularly for smaller businesses or those lacking the necessary infrastructure to comply with regulatory requirements.
What are some potential future developments in the regulatory landscape for Weed Schedule 3?
+The future of Weed Schedule 3 regulations may involve ongoing advancements in cannabis research, leading to the refinement or adjustment of existing criteria. Shifting societal attitudes and policy changes may also influence the regulatory landscape, potentially leading to more flexible regulations or the exploration of alternative classification systems. International collaborations and harmonization efforts could further shape the future of Weed Schedule 3, ensuring a consistent and evidence-based approach worldwide.


